Feline injection-site sarcomas (FISS) occur with a prevalence of up to 16 per 10,000 cases, and the proportion of ISS compared to non-ISS has increased over the years (Liptak and Christensen, 2019).
The term injection-site sarcoma is preferred over vaccine-associated sarcoma as FISSs have been reported to arise at sites of injections other than those of vaccines. There does not appear to be a particular vaccine or manufacturer more significantly associated with ISS development, and factors such as needle gauge, syringe type or mixing of vaccines do not influence risk (Kass et al., 2003). It is currently unclear whether vaccines containing adjuvant (eg aluminium hydroxide) increase ISS risk; however, it is thought that adjuvant-containing vaccines may increase local reaction following administration (Macy and Hendrick, 1996).
Pathology
The development of ISS is likely linked to the local inflammatory response following injection, which leads to uncontrolled fibroblast and myofibroblast growth and eventual tumour formation. This is supported histologically by evidence of transitions between inflammation and sarcoma, and small foci of sarcoma within peripheral inflammatory tissue (Esplin et al., 1993; Hendrick and Brooks, 1994).
Feline injection-site sarcomas are aggressive tumours and, histologically, demonstrate features such as high mitotic activity, marked cellular pleomorphism and increased necrosis
Macrophages in the surrounding inflammation often contain aluminium and oxygen, appearing as bluish-grey foreign material on histology (McNiel, 2001). Feline ISSs are aggressive tumours and, histologically, demonstrate features such as high mitotic activity, marked cellular pleomorphism and increased necrosis; almost 60 percent are high-grade (grade III) tumours (Phelps et al., 2011).
Presentation and diagnosis
FISSs often present as irregular subcutaneous masses at sites of previous injections, typically in the interscapular region. They can develop anywhere between 4 weeks and 10 years post-injection and can demonstrate rapid growth (Macy and Hendrick, 1996).
As for most cutaneous and subcutaneous masses, fine needle aspirate cytology is initially indicated for diagnosis. However, sarcomas do not exfoliate cells readily, which can make definitive diagnosis challenging. Cytology may only be “suggestive” of sarcoma but should at least rule out round cell or epithelial tumours. Biopsy for histopathology may therefore be required for definitive preoperative diagnosis. If a biopsy is obtained, it is important that the biopsy tract is excisable alongside the mass itself, without increasing surgical margins.

Once FISS is diagnosed or clinically suspected, further investigation is required before considering treatment options. A minimum database consisting of haematology, biochemistry and urinalysis should be performed. As surgery is the mainstay of treatment for ISS, and a successful outcome relies heavily on wide and complete excision, advanced imaging to better visualise the extent of the tumour is crucial.
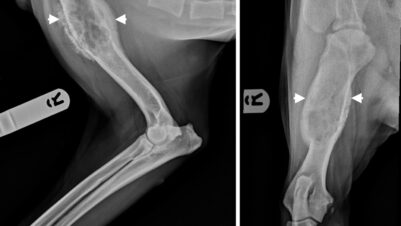
FISSs typically have long finger-like projections lateral and deep to the main mass and can also have smaller “satellite” nodules peripheral to the primary tumour. Contrast-enhanced computed tomography (CT), or less commonly MRI, helps visualise these and aid surgical planning (Figure 1). For interscapular ISS, post-contrast CT scans should be performed with the forelimbs extended both cranially and caudally along the body, as this allows a better understanding of the relationship between the tumour and adjacent tissue (Longo et al., 2018). As the surgical dose for ISSs can be large, imaging should include the abdominal and thoracic cavities to screen for comorbidities that may preclude a more invasive treatment approach.
Imaging also assesses for metastatic disease, although the rate of metastasis is relatively low for ISS; if metastasis does occur, it is primarily to the lungs. Rates are reported between 0 and 24 percent, although this does vary depending on tumour grade (Liptak and Christensen, 2019).
Treatment
Surgery
Outcomes can be good for FISS, with high rates of local control, as long as wide complete excision is achieved. It is therefore important that the first surgery is performed by a surgeon who is experienced in aggressive resection of ISS. Studies have shown significantly reduced progression-free intervals (PFI) and survival times in cats when surgery is performed in a non-referral setting (Hershey et al., 2000).
Currently, it is advised to resect ISSs with 5cm lateral margins and two uninvolved fascial planes as the deep margin, although this can be reduced to 3cm lateral margins if based on CT imaging. This results in complete excision in 95 to 97 percent of cases compared to 50 percent when only 3cm lateral margins (based on palpation) are obtained (Davidson et al., 1997; Hershey et al., 2000; Phelps et al., 2011; Romanelli et al., 2008). For ISSs in the interscapular region, surgery often involves the excision of dorsal spinous processes and the tips of the scapula, whereas body wall resection or amputation are usually required for ISSs of the flank and extremities, respectively (Figure 2).

Even with these more aggressive surgical doses, the incidence of major post-operative complications is relatively low; for example, wound dehiscence is reported in 11 to 17 percent of cats (Cantatore et al., 2014; Phelps et al., 2011).
Even following complete excision, local recurrence can occur in 14 to 22 percent of cats. However, this is significantly increased (58 to 69 percent) in cats with incompletely excised ISS (Bray and Polton, 2016; Giudice et al., 2010; Phelps et al., 2011; Poirier et al., 2002). Median survival times (MST) can be prolonged following complete excision – reported between 804 days to not reached (Bray and Polton, 2016; Phelps et al., 2011; Romanelli et al., 2008). This is shorter for cats with incomplete excision, where median survival times between 395 and 608 days are described (Davidson et al., 1997; Dillon et al., 2005; Hershey et al., 2000).
Radiation therapy
Where aggressive local resection is not possible or incomplete excision is encountered despite surgery, adjuvant radiation therapy (RT) is often recommended.
Recurrence rates for FISS treated with surgery and adjuvant RT are reported between 29 and 41 percent, lower than the 58 to 69 percent described for incompletely excised FISS treated with surgery alone (Bregazzi et al., 2001; Rossi et al., 2019; Cohen et al., 2001). However, the quality and amount of literature is limited, and further studies are needed to better assess the efficacy of adjuvant RT and guide optimum protocol selection. If RT is elected, one study demonstrated improved PFI and MST if started within 10 to 14 days post-operatively (Cohen et al., 2001).
Where aggressive local resection is not possible or incomplete excision is encountered despite surgery, adjuvant radiation therapy is often recommended
For non-resectable ISS, RT can be considered in the macroscopic setting. However, the duration of response is often short-lived, and it is therefore more of a palliative treatment approach. Studies have shown 20 percent and 70 percent of cats achieve a complete or partial response to treatment, respectively, with PFIs between four and eight months and MSTs between seven and ten months (Eckstein et al., 2009; Kleiter et al., 2010; Nolan et al., 2013).
Other treatment modalities
Adjuvant systemic chemotherapy is rarely used for cats with ISS as the risk of metastasis is relatively low, and most studies show no improvement in survival when it is administered following surgical excision (Liptak and Christensen, 2019). However, one study did demonstrate improvement in time to tumour recurrence in cats treated with adjuvant doxorubicin (Poirier et al., 2002).
Chemotherapy is perhaps more indicated in the macroscopic disease setting where surgery or RT are declined. Doxorubicin with or without cyclophosphamide is most commonly used. Up to 50 percent of cats will demonstrate a complete or partial response to this treatment; however, the duration of response is often short, with median PFI reported between 83 and 125 days (Barber et al., 2000; Poirier et al., 2002).
Electrochemotherapy (ECT) involving intratumoral or systemic chemotherapy administration followed by the application of electrical impulses to the tumour or scar is a generally well-tolerated treatment that is gaining popularity in veterinary oncology. Although adjuvant RT has a wider evidence base, one study assessing post-operative ECT for incompletely excised FISS showed a low recurrence rate of 18.5 percent with the median PFI not reached and median survival time of 1,000 days; treatment consisted of two ECT sessions (Spugnini et al., 2020). Adjuvant ECT could therefore be a consideration for patients with increased anaesthetic risk or where owners decline RT for other reasons (eg logistics and finance).
Guidelines for prevention
Numerous recommendations for preventing FISS have been discussed, including avoiding vaccines with aluminium-based adjuvants, using non-adjuvanted vaccines, avoiding polyvalent vaccines and increasing vaccine interval; however, these remain controversial (Liptak and Christensen, 2019).
Although not aimed at reducing the risk of feline injection-site sarcomas, the simplest change that can improve the management and likely outcome […] is administering vaccines (or other injections) in alternative sites
Although not aimed at reducing the risk of FISS, the simplest change that can improve the management and likely outcome of ISS in individual patients is administering vaccines (or other injections) in alternative sites. As wide and complete surgical excision is the most important aspect of ISS management, injecting in sites more amenable to wide excision is beneficial. It is therefore strongly recommended to administer injections in distal limbs or the tail, as limb or tail amputations are simple and likely to achieve complete wide excision compared to interscapular ISS.