Senior pet nutrition is a growing focus as pets in the UK live longer and we recognise the key role nutrition can play in health. There are various definitions as to the age a pet becomes classified as “senior” and these are largely subjective. Generally, cats are said to be senior from age 11 (ISFM, 2018). In dogs, it varies according to breed, with larger breeds classed as senior at around five to eight years and small breed dogs from the age of 10 (FEDIAF, 2017).
Ageing involves progressive physiological and metabolic changes leading to a decrease in organ function, and is influenced by, for example: breed size, genetics, environmental factors and nutritional factors. Functional and physiological changes associated with ageing include alterations in energy requirements (with a decreased metabolic energy requirement in dogs but no similar reduction in cats), decreased digestive and absorptive ability (most notable in cats) and sarcopenia.
Sarcopenia and the role of protein
Unless a condition requiring protein restriction, such as renal disease, is present, protein restriction is believed to cause harm and may be even more damaging than in young animals (Churchill, 2018). In fact, protein requirements are increased in the senior life stage compared to adults. In dogs, this occurs due to an imbalance in the rate of turnover, with increased protein degradation and reduced protein synthesis (Churchill, 2018); in cats, impaired digestive ability also contributes (Williams, 2018).

Sarcopenia is a reduction in lean muscle mass unrelated to disease which becomes more evident in the senior pet. It is of particular significance since it increases the risks for morbidity and mortality (Laflamme, 2018). This should be differentiated from cachexia, which involves muscle wastage associated with a disease state, though this also contributes to morbidity and mortality (see Figure 1). In apparently healthy cats, approximately one third of lean body mass (LBM) is lost from the age of 10 to 15 years (Laflamme, 2018). Senior dogs lose approximately 10 percent LBM and experience a 10 percent increase in their fat mass over the same period of time – something which could make canine sarcopenia challenging to identify if muscle wastage is hidden under excess fat stores.
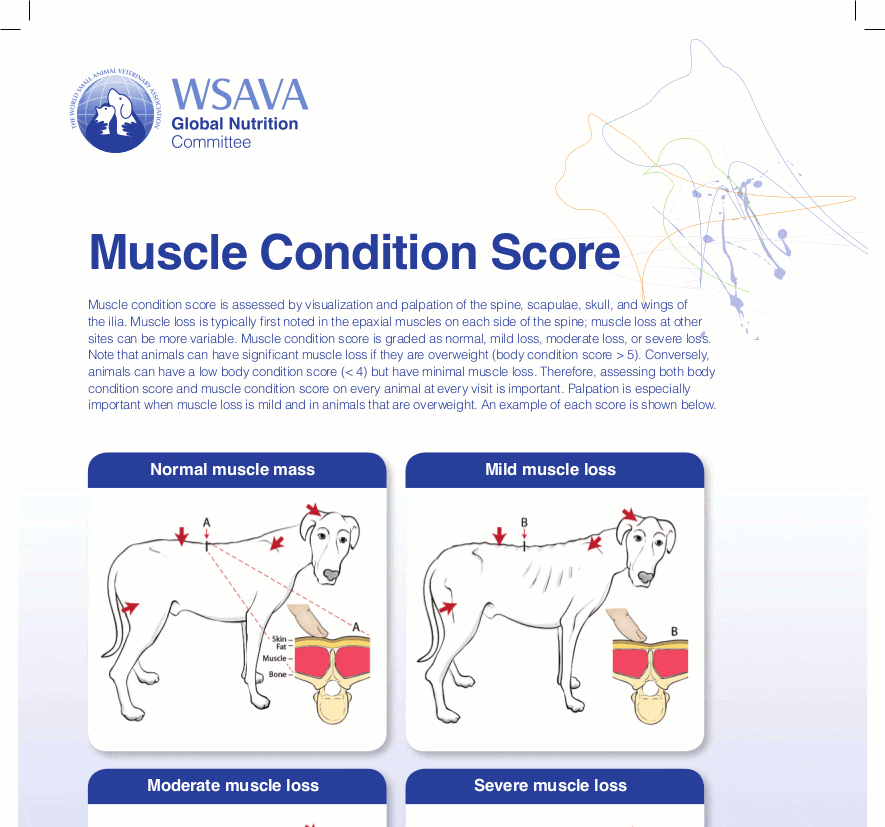
Given the increased awareness of the impact sarcopenia can have on health and lifespan, clinicians should monitor this regularly in their senior patients. Muscle condition scoring (MCS) charts (Figure 2) can be a very useful tool to aid this and are free to download from the World Small Animal Veterinary Association (WSAVA) website. MCS is subjective, with classifications of normal, mild, moderate and severe. A score is reached by both visually assessing and palpating the patient, with particular focus on the bony prominences and epaxial muscles, since these tend to be the earliest sites of muscle loss in both dogs and cats (WSAVA, 2018). A proactive and preventative approach is preferred, particularly since, once lost, it is more difficult to regain muscle than fat.
What is thought to cause sarcopenia, and how can it be influenced if detected? More research has been done in humans than companion animals, but it appears to have a multifactorial aetiology (Figure 3). It is believed that several management factors; importantly, physical exercise, but also nutrition, can minimise its incidence and/or severity.

When dietary protein intake is inadequate, protein will initially be depleted from skeletal muscle, accelerating muscle wastage. General guidelines of 2.55g protein/kg bodyweight (BW) for healthy dogs and 5g/kg BW for cats have been suggested, but senior animals may need up to 50 percent more than this (Churchill, 2018). Although still an ongoing area of investigation, an increased intake of protein appears to be of particular benefit and has demonstrated a reduction in sarcopenia in dogs and cats (Laflamme, 2018). As yet, however, no optimal protein level has been determined.
As well as the overall level of dietary protein, particular types of protein and amino acids may also impact LBM. The anabolic effect of protein seems to be reliant on a threshold concentration being reached and faster absorbed proteins appear able to reach this threshold better. Whey, a more rapidly absorbed protein, is thought to contribute to greater protein synthesis (Laflamme, 2018). The amino acid lysine has also shown protection of LBM in both dogs and cats in preliminary studies (Laflamme, 2018). However, more research is required to better elucidate how additional dietary protein may improve muscle mass, and the amount and source(s) which may offer most benefit.
Other nutrients of interest with relation to sarcopenia
Other dietary factors which may influence sarcopenia include vitamin D and omega 3 fatty acids. Vitamin D can affect gene transcription, consequently affecting muscle cell metabolism. There appear to be links between vitamin D supplementation in humans and an increase in muscle mass and strength, though more research is needed (Laflamme, 2018). Omega 3 fatty acids in fish oil are well known to be able to reduce inflammatory mediators. Inflammatory mediators are commonly seen in sarcopenic humans and may be present in sarcopenic pets. Some interventional studies in humans do demonstrate correlations between increased omega 3 fatty acids and muscle mass, but no data currently exists evaluating the impact of fish oil in muscle mass or function in companion animals.

Feline enteropathies
Cats show a negative correlation between age and protein digestibility, and a fifth of cats aged over 14 have impairments of protein digestibility of greater than 20 percent (Figure 4). The same is not thought to be true of dogs. In cats, increased amounts of protein within the diet may thus be beneficial not only to minimise sarcopenia but also to compensate for reduced protein digestibility.
Although the cause(s) of this poorer digestive ability is still unknown, an idiopathic chronic enteropathy (ICE) is believed to play a role in many cases. Indeed, many cats with impaired protein digestion also show a reduction in their ability to digest fat. This may therefore contribute to both sarcopenia and a reduction in body weight (which tends to be seen in cats over the age of 11 years). Although some cats may compensate for this by eating more, thus not exhibiting weight loss or sarcopenia, in others this is not the case (Williams, 2018).
Protein intake is dependent on overall food intake as well as the food’s composition, and is closely linked to energy intake, so if patients do not meet their energy requirement they will be unable to meet their protein needs. Therefore, when considering diet, many geriatric cats may benefit from both an increased energy level and protein content within their diet in order to compensate for poorer digestive ability and no reduction in metabolic energy requirements.
In comparison, older dogs show a reduction in metabolic energy requirements of approximately 25 percent. Thus, although they may benefit from increased protein levels to minimise the incidence of sarcopenia, they tend to require a food of a lower energy density. For many senior canines, therefore, a diet with a higher protein:calorie ratio is likely to be of benefit (Churchill, 2018). These are, however, generalisations only and a comprehensive nutritional assessment should be undertaken in all individuals during a senior health screen to tailor nutritional advice to the specific patient and clinical case.
A full reference list is available on request











