In companion animals, parasitic infections remain a pressing health concern, impairing digestive health, skin integrity, immune competence and physiological balance. While antiparasitic therapies and preventative protocols are central to managing parasite infections, the contribution of nutrition to host resistance and recovery is becoming more evident, despite remaining underutilised in clinical practice. The interplay between parasitic disease, nutritional status and immunity, though complex and not yet fully understood, has been documented across several conditions. For example, dogs infected with the blood parasite Babesia rossi exhibit significantly reduced vitamin D levels, a deficiency closely linked to increased disease severity (Mellanby et al., 2019). Vitamin D plays a significant immunomodulatory role by binding to the vitamin D receptor, which is expressed on various antigen-presenting cells (essential for regulating the immune response to infections), including monocytes, macrophages and dendritic cells (Baeke et al., 2010).
Micronutrient deficiencies and parasite impact
Intestinal protozoa such as Giardia spp. and Cystoisospora spp. can lead to malabsorption and nutrient deficiencies, including lower levels of urea, total protein, albumin, globulin and cholesterol. Importantly, vitamin B12 deficiency affects 73.7 percent of dogs infected with Giardia, 88.8 percent of dogs infected with Cystoisospora and 100 percent of dogs co-infected with both parasite species (Vatnikov et al., 2020).
Zinc, an essential mineral for both innate and adaptive immunity, has been shown to enhance pharmacological treatment, accelerating clinical recovery and reducing relapse rates in several cases of canine leishmaniosis
Beyond these associations, micronutrient supplementation has demonstrated therapeutic potential. Zinc, an essential mineral for both innate and adaptive immunity, has been shown to enhance pharmacological treatment, accelerating clinical recovery and reducing relapse rates in several cases of canine leishmaniosis (CanL) (Paradies et al., 2017; Prasad, 2009; Hojyo and Fukada, 2016). Vitamin D deficiency, conversely, is correlated with disease progression (Rodriguez-Cortes et al., 2017). Supplementation with vitamins A and D, along with zinc, has also been found to increase parasite-killing activity via upregulation of nitric oxide and reactive oxygen species (ROS) in immune cells (Hernandez et al., 2021).
Antioxidant and vitamin supplementation in recovery
Antioxidant supplementation with vitamin E and selenium can enhance the clinical recovery of dogs affected by mange mites, ticks and fleas when used alongside standard antiparasitic treatments (Kubesy et al., 2020). This supplementation leads to significant improvements in oxidative stress biomarkers, including increased catalase activity and decreased malondialdehyde (MDA) levels in the mange and tick- and flea-infested groups (Kubesy et al., 2020). Vitamin E and selenium can therefore be considered as complementary therapies to support the standard treatment for external parasitism in dogs.
The host–parasite–nutrition triad
This framework highlights how malnutrition increases susceptibility to infection and impairs recovery, while parasitic infections compromise nutrient availability and immune function
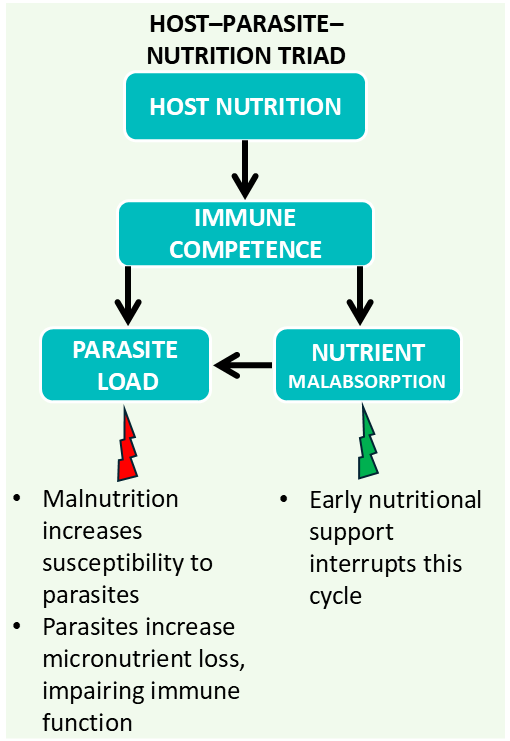
To better understand how nutrition can influence the course and outcome of parasitic diseases in companion animals, it is important to consider the interdependent relationship between the animal host, the parasite and the nutritional status of the host. This dynamic interaction is known as the host–parasite–nutrition triad. This framework highlights how malnutrition increases susceptibility to infection and impairs recovery, while parasitic infections compromise nutrient availability and immune function (Figure 1).
Clinical cases: parasitic diseases and micronutrient deficiencies
To illustrate the various ways in which parasitic infections affect nutritional status and recovery in companion animals, the following sections examine specific parasitic diseases. Each case highlights different scenarios of nutrient depletion and the potential for targeted supplementation to improve clinical outcomes (Table 1).
| Parasitic disease | Nutritional impact | Supplementation benefit |
| Giardiasis | Decreases vitamin B12 (73.7 percent), protein and cholesterol | Unknown |
| Cystoisosporiasis | Decreases vitamin B12 (88.8 percent), protein and cholesterol | Unknown |
| Co-infection (Giardia and Cystoisospora) | Decreases vitamin B12 (100 percent) | Unknown |
| Canine leishmaniosis | Decreases zinc and vitamin D | Zinc and vitamins A and D reduce relapse and support immunity |
| Babesiosis | Decreases vitamin D, iron, zinc and copper | Supplementation may improve anaemia and reduce oxidative stress |
| Sarcoptic mange | Decreases zinc, copper, iron and vitamins A and C | Vitamin E and selenium decrease oxidative stress and improve clinical recovery |
| Canine demodicosis | Decreases zinc and copper, and increases iron | Vitamin C and beta-carotene restore antioxidant balance |
| Spirocercosis | Decreases vitamin D (linked to neoplasia progression) | Vitamin D shows therapeutic potential (but needs more research) |
Leishmaniosis
CanL is a chronic zoonotic disease caused by Leishmania infantum in which domestic dogs act as the primary reservoir, transmitting the parasite to humans through phlebotomine sandflies. CanL is characterised by a wide range of clinical manifestations and immunopathological effects, making its diagnosis and treatment challenging. Despite ongoing control efforts, the disease remains endemic in various regions in Europe and continues to pose a significant public health risk due to its persistence and difficulties in treatment (Solano-Gallego et al., 2009). Treatment failure further compounds the issue, as infected dogs continue to serve as reservoirs, increasing the burden on public health.
A major feature in the development and progression of CanL is immune imbalance. Infected dogs typically show a suppressed Th1 immune response, which is responsible for eliminating intracellular pathogens, alongside a dominant and less effective Th2 response (Lima et al., 2012). This immune imbalance facilitates the persistence of the parasite. Regulatory T cells exacerbate this imbalance by producing interleukin-10 (IL-10), a cytokine that weakens protective immunity, further hindering the dog’s ability to clear the parasite (Silva et al., 2014).
Impact of supplementation on leishmaniosis
Emerging evidence suggests that nutritional interventions may help support immune responses in CanL
Emerging evidence suggests that nutritional interventions may help support immune responses in CanL. Previous research has shown that the addition of specific nutrients, such as vitamins A and D and zinc, to spleen white blood cell cultures from infected dogs enhances immune activity and reduces parasite burden (Hernandez et al., 2021). These nutritional supplements may help correct the immune imbalance associated with CanL.
Research in dogs with visceral leishmaniosis (VL), another form of the disease caused by L. infantum, highlights the importance of vitamin D. Vitamin D deficiency has been strongly linked to the progression of VL, suggesting a crucial role of the vitamin D pathway in the immune response against the parasite (Rodriguez-Cortes et al., 2017). To further support this conclusion, supplementing with vitamin D has demonstrated benefits in mitigating the symptoms and progression of VL in naturally infected dogs (Rodriguez-Cortes et al., 2017). Therefore, supplementing vitamin D in dogs with infection has the potential to enhance immune function and decrease disease susceptibility.
The addition of nutritional supplements, such as vitamins A and D and zinc, shows promise in enhancing treatment outcomes, reducing relapse and decreasing the zoonotic risk posed by infected dogs. Vitamins A and D and zinc supplementation can reduce IL-10 levels, a potent anti-inflammatory and immunosuppressive cytokine, and increase the production of tumour necrosis factor-alpha (TNF-α), a pro-inflammatory cytokine associated with effective parasite clearance (Gradoni, 2015; Hernandez et al., 2021). These findings support the potential of targeted supplementation to improve treatment efficacy in CanL. Although interferon gamma (IFN-γ) is typically protective, elevated levels of this cytokine, alongside high IL-10 and parasite overload, suggests that immune responses must be not only strong but also well regulated and balanced for successful parasite clearance (Corrêa et al., 2007; Lage et al., 2007).
A study by de Sousa Gonçalves et al. (2021) demonstrated that nutritional intervention, specifically the supplementation of omega-3 polyunsaturated fatty acids and B vitamins, prior to anti-Leishmania drug treatment resulted in better clinical outcomes in dogs with CanL, including improvement in renal function and reductions in lipid and protein peroxidation. Importantly, the addition of these nutritional adjuvants, starting 30 days before treatment, controlled inflammation, as evidenced by reduced inflammation markers and improved serum protein ratios.
Collectively, these results support the potential role of nutritional supplementation in the treatment and management of CanL. When introduced early, such interventions may help regulate the immune response and improve clinical outcomes. However, it is important to note that current evidence is based on preliminary data and has not yet been incorporated into clinical guidelines.
Babesiosis
Similar to leishmaniosis, babesiosis in dogs is also marked by immune disruption and micronutrient imbalances, particularly affecting iron and trace minerals critical for recovery. In canine babesiosis, significant reductions in dietary intake, often manifested as anorexia and substantial weight loss, take place. This impact on nutrition is particularly evident in the levels of essential vitamins and minerals, with dogs naturally infected with this haemoprotozoan parasite showing marked reductions in blood levels of key micronutrients (Chaudhuri et al., 2008; Dvir et al., 2019).
Hypovitaminosis D has been identified in association with babesiosis (Dvir et al., 2019), which suggests that low vitamin D could contribute to the development of this infection through vitamin D deficiency. Additionally, dogs infected with Babesia also exhibit significantly lower levels of iron, zinc and copper in their blood compared to healthy dogs (Chaudhuri et al., 2008).
These deficiencies are of clinical concern due to the critical roles these micronutrients play in maintaining essential physiological functions. Iron is vital for haemoglobin production, and its reduction directly contributes to the anaemia frequently seen in babesiosis cases. Zinc and copper are essential for maintaining the body’s antioxidant defence systems. Deficiencies in these two minerals impair the body’s ability to cope with oxidative stress, which is elevated during infection.
The combined effect of iron, zinc and copper deficiencies exacerbates the severity of both anaemia and oxidative damage in dogs infected with babesiosis. These deficiencies impair the body’s ability to handle the stress induced by the infection, potentially prolonging disease duration and complicating the treatment and recovery process.
These findings underscore the critical role micronutrient deficiencies play in the progression and complications of canine babesiosis. Addressing these deficiencies through nutritional interventions or supplementation to restore normal levels of essential micronutrients could help improve treatment outcomes.
Spirocercosis
Similar to other parasitic infections, spirocercosis impacts immune defence mechanisms of the host. However, spirocercosis introduces additional complexity by linking nutritional status, especially vitamin D deficiency, with increased cancer risk. Spirocercosis in dogs is caused by the roundworm Spirocerca lupi. The disease is characterised by the formation of oesophageal nodules, which can undergo neoplastic transformation. This transformation may lead to the development of osteosarcoma, fibrosarcoma or undifferentiated sarcoma. The disease progression into a neoplastic state has significant implications for the dog’s health and prognosis.
Although it remains unclear whether vitamin D supplementation could directly influence the course or severity of spirocercosis, supplementation could be considered as part of a broader therapeutic strategy
A previous study has shown that dogs with spirocercosis have significantly lower serum vitamin D levels compared to healthy dogs. This reduction in vitamin D is particularly significant in the neoplastic form of the disease. As the disease progresses from the non-neoplastic to the neoplastic stage, a further decline in vitamin D levels is observed (Rosa et al., 2013). These findings suggest that low vitamin D status may play a role in the neoplastic transformation of spirocercosis in dogs.
Although it remains unclear whether vitamin D supplementation could directly influence the course or severity of spirocercosis, supplementation could be considered as part of a broader therapeutic strategy. Given the observed relationship between low vitamin D levels and the neoplastic progression of spirocercosis, supplementation might offer some benefit, but it should be implemented alongside other treatments specifically tailored to manage the disease.
Sarcoptic mange
Unlike systemic parasitic diseases, ectoparasitic infections such as sarcoptic mange primarily target the skin but can also produce systemic effects through nutrient depletion and oxidative stress. Sarcoptic mange in dogs, a highly contagious skin infection, is caused by Sarcoptes scabiei var. canis. It is characterised by various dermatological symptoms such as intense pruritus, vesiculo-papular eruptions, pinpoint crusts and alopecia. In addition to dermatological symptoms, affected dogs show haematological changes, including reduced haemoglobin, haematocrit and erythrocyte count, and increased leucocyte counts, neutrophilia and lymphopenia. These changes indicate systemic involvement, affecting various biological functions beyond the skin (Beigh et al., 2013a, 2016).
The infestation leads to significant alterations in oxidative stress, trace element concentrations and vitamin levels, which can affect various biological systems beyond the skin. Dogs affected by sarcoptic mange exhibit significant reductions in plasma concentrations of zinc, copper and iron, with the most severe deficiencies observed in dogs with intense infestations (Beigh et al., 2013a, 2016). Zinc deficiency has also been reported in dogs with follicular mange (Dimri et al., 2008a). Moreover, the levels of vitamins A and C were significantly decreased in infested dogs, potentially compromising their immune response and overall health. These deficiencies contribute to both the local and the systemic effects of the infestation.
One of the key underlying mechanisms of these changes is oxidative stress. MDA, a marker of oxidative damage, is significantly elevated in severely infested dogs, indicating cell and tissue damage. In contrast, the activity of important antioxidant enzymes, such as superoxide dismutase and catalase, is significantly reduced, suggesting an imbalance between oxidants and antioxidants (Beigh et al., 2016).
Impact of mineral deficiency on sarcoptic mange
Zinc plays a crucial role in mitigating oxidative damage by scavenging ROS generated during the infection (Ewans and Halliwell, 2001). However, in dogs with sarcoptic mange, zinc is rapidly depleted as it is utilised to counteract the excess ROS, thereby contributing to systemic oxidative stress. This depletion of zinc likely exacerbates both the skin lesions and the systemic abnormalities observed, indicating a significant link between zinc deficiency and the severity of sarcoptic mange.
These interconnected factors highlight the need for careful monitoring and intervention to address trace element deficiencies in dogs with sarcoptic mange.
The depletion of zinc has further implications for erythropoiesis, the process of red blood cell production. Toxic substances secreted by the parasitic mites likely suppress erythropoiesis (Deloach and Wright, 1981), while oxidative damage accelerates the destruction of erythrocytes, contributing to the observed reductions in haemoglobin, haematocrit and erythrocyte count (Al-Qudah et al., 2010). In addition to zinc deficiency, vitamin A plays a vital role in erythropoiesis. Its deficiency impairs the production of healthy red blood cells, leading to the production of deformed erythrocytes, which are removed by macrophages in the reticuloendothelial system. As a result, the deficiency of vitamin A contributes to the observed anaemia in dogs with sarcoptic mange (Roodenburg et al., 2000).
The combined effects of zinc depletion, oxidative stress and vitamin A deficiency have a compounded impact on both the skin and the haematological health of affected dogs. These interconnected factors highlight the need for careful monitoring and intervention to address trace element deficiencies in dogs with sarcoptic mange. Therapeutic strategies should not only focus on managing the parasitic infestation but also aim to restore the balance of essential nutrients to support skin integrity, immune function and normal blood parameters.
A previous study demonstrated that a combination of ivermectin and vitamin E-selenium supplementation significantly improves clinical recovery in dogs infested with Sarcoptes scabiei (Behera et al., 2011). The addition of these antioxidants to the standard ivermectin treatment not only enhances clinical outcomes but also helps normalise oxidative stress parameters, including haematological values and antioxidant enzyme activities. This combination therapy accelerates recovery compared to ivermectin alone, making it a recommended adjunct treatment for managing canine sarcoptic mange.
Sarcoptic mange in dogs induces significant biochemical and haematological disruptions, including deficiencies in trace elements such as zinc and copper, oxidative stress and vitamin A depletion. These changes underscore the need for a multifaceted treatment approach, integrating both antiparasitic therapy and nutritional support, to restore overall health and improve clinical outcomes in affected dogs.
Canine demodicosis
Another skin-related parasitic condition, canine demodicosis, shares many of the oxidative and micronutrient imbalances seen in sarcoptic mange, but often presents with more chronic and generalised symptoms. Canine demodicosis is caused by the proliferation of Demodex canis in the hair follicles and sebaceous glands, making it the most common and serious form of canine dermatosis. This condition can lead to a range of clinical manifestations and is particularly concerning when it progresses to generalised demodicosis.
Generalised demodicosis in dogs is associated with significant alterations in the levels of trace elements and antioxidants. Specifically, there is a marked reduction in zinc and copper, alongside a significant increase in iron (Beigh et al., 2013b). The lower levels of zinc and copper may be linked to their overutilisation in the synthesis of antioxidant enzymes that help counteract oxidative stress caused by the disease.
In dogs with demodicosis, the levels of the oxidative damage marker MDA were found to be significantly higher. Conversely, the activity of key antioxidant enzymes, superoxide dismutase and catalase, was significantly lower. This reflects an imbalance between oxidants and antioxidants, leading to increased oxidative stress, which exacerbates the condition.
Additionally, beta-carotene and vitamin C levels were significantly lower in diseased dogs, further contributing to the compromised antioxidant defences. These alterations are likely secondary to the changes induced by demodectic mange, reinforcing the oxidative damage that the body experiences during the disease.
The findings highlight the role of oxidative stress and trace element imbalances in the pathophysiology of canine demodicosis. The reduction in zinc and copper, combined with an increase in iron and a disrupted oxidant/antioxidant balance, may worsen the clinical manifestations of the disease. Addressing these imbalances through nutritional or therapeutic interventions could be beneficial in managing canine demodicosis.
Conclusion
Current evidence underscores the potential of micronutrient supplementation as a promising adjunct to existing antiparasitic therapies
Parasitic infections in companion animals pose significant challenges, but nutrition plays a crucial role in both prevention and recovery. A balanced diet rich in immune-supportive nutrients and easily digestible ingredients can help animals resist infections, recover more quickly and maintain their overall health. Although nutrition is not a cure for parasitic diseases, it supports immunity, slows disease progression and enhances recovery. Micronutrient supplementation, particularly with vitamins A, D, E and B12, zinc and selenium, has shown promise in improving clinical outcomes by strengthening immune function and reducing inflammation.
Nutrition should be regarded as a key component of a comprehensive parasite management strategy. When combined with dewormers, flea and tick preventatives, and good hygiene practices, it becomes a powerful ally in controlling parasitic infections. While further studies are required to fully understand the host–parasite–nutrition triad and to decipher mechanisms by which micronutrient supplementation influences parasitic infections, current evidence underscores the potential of micronutrient supplementation as a promising adjunct to existing antiparasitic therapies.








